nermin.demirkol15.03.2010_10.43.46sci

Ceramics International 32 (2006) 325–330

www.elsevier.com/locate/ceramint
![]()
Abstract
Influence of mixing/milling on sintering and technological properties of anorthite based porcelainised stoneware
M.U. Taskiran a,b, N. Demirkol a, A. Capoglu a,*
a Department of Material Science and Engineering, Gebze Institute of Technology, P.O. Box 141, 41400 Gebze, Kocaeli, Turkey
b KALESERAMIK, Canakkale Kalebodur Ceramic Plant, Canakkale, Turkey
Received 25 November 2004; received in revised form 15 December 2004; accepted 4 March 2005
Available online 17 May 2005
The effect of mixing/milling time on sintering and technological properties of a new porcelainised stoneware body was investigated. This stoneware body is basically based on anorthite (CaO·Al2O3·2SiO2) crystals development in the microstructure and was fabricated by using wollastonite, calcined alumina, quartz, Ukrainian ball clay and some magnesia as raw materials. Laboratory scale tests were carried out starting from mixing/milling of a reference body composition for different durations (3, 12, 24, 48 and 96 h) in a ball mill. Sintering and technological properties such as density, water absorption, firing shrinkage, flexural strength of each body were measured. X-ray diffraction (XRD) and scanning electron microscopy (SEM) studies were carried out to analyse the microstructure.
It was found that mixing/milling has a major effect on the particle size distribution, which in turn controls particles packing. High degree of close particle packing increases the densification by reducing the number and size of closed porosity in fired microstructure, which in turn contributes to the improvement of flexural strength.
# 2005 Elsevier Ltd and Techna Group S.r.l. All rights reserved.
Keywords: Mixing/milling; Vitrified; Anorthite; Porcelainised stoneware
![]()
1. Introduction
Porcelainised stoneware tiles are vitrified, unglazed ceramics used extensively in constructions as flooring and covering material for decorative purposes. Recently, an alternative composition for making porcelainised stoneware was developed by Taskiran and co-workers [1,2]. This new body is designed to develop basically anorthite (CaO·Al2O3·2SiO2) crystals in the microstructure and to have a high crystalline to glassy phase ratio, after processing at 1200–1225 8C temperature ranges. To obtain the anorthite crystals, wollastonite, calcined alumina, quartz, Ukrainian ball clay and some magnesia were used as raw materials each with its distinct initial particle size distribution. After a number of process operations such as crushing, grinding and![]()
* Corresponding author. Tel.: +90 262 6538497x1080;
fax: +90 262 6538490.
E-mail address: capoglu@penta.gyte.edu.tr (A. Capoglu).
dispersion, a suitable particle size distribution of the mixed material is attained. The final properties of the ceramic product are mainly limited by the presence of various types of defects, which can arise from agglomerates present in the early stages of processing and these may lead to packing inhomogeneity during the forming process [3]. It is widely recognised that the particle size distribution in a body mixture has a significant impact on the packing efficiency, which in turn influences the size and shape of pores, the shrinkage behaviour and microstructure development.
Process control is a key concept in managing the industrial process to prevent errors and problems rather than relying on post process activities, such as inspection and sorting out defective products [4]. Uniformity concept in ceramic processing dominates every aspect of ceramic processing. The concept emphasizes the need to obtain uniformity of the characteristic properties on small and large scales of sizes at every stage of ceramic processing. The employment of uniformity concept and uniform heat
0272-8842/$30.00 # 2005 Elsevier Ltd and Techna Group S.r.l. All rights reserved. doi:10.1016/j.ceramint.2005.03.010
326
M.U. Taskiran et al. / Ceramics International 32 (2006) 325–330
treatment in processing of ceramic objects would develop uniform characteristic properties. In order to describe the uniformity concept, the non-uniformity approach could be adopted since poor mixing/milling must manifest itself in the behavioural properties of the final product. This approach has been discussed by Messer and co-workers [5–8] in which he indicated that a detailed examination of the microstructure of a ceramic material can reveal non- uniformity of porosity and grain size, which in turn reflects the non-uniform distribution of the components resulting from the mixing operation. Such observations, he pointed out, could be used in conjunction with measurement of relevant behavioural properties to provide a reasonable indication of the efficiency of the mixing operation. Lee and Iqbal [9] studied the effect of mixing on mullite formation in porcelain and they found that the size, shape and composition of mullite crystals are largely determined by the extent of mixing of the original powder batch.
Mixing/milling is a crucial step in ceramic processing [10] because it brings about an improvement in the chemical and physical uniformity of a powder mixture. If ordering and demixing do not occur during and after the mixing operation, the best mixture that can be obtained is a random homogeneous mixture. Unlike a homogeneous mixture whose composition does not vary with position, the type of particle at a given position in a random homogeneous mixture is determined by chance.
One method of improving the packing efficiency in a particulate mixture is to obtain size distributions for the various components so that the overall body may be considered as consisting of a binary mixture of coarse and fine components. Previous theoretical [11–14] and experi- mental studies [15,16] have shown that appropriate size distributions of the particles in ceramic bodies promote the particle packing in the shaped object and, hence, increase the apparent density in the green state. It was demonstrated for SiC [17] and Al2O3, [18] that it is possible to obtain high density slip cast bodies by using bimodal particle size distributions in which fine and coarse powders are combined in an appropriate proportion and size ratio.
In the case of traditional ceramics, the coarse component is composed of filler and flux, each with a median size of about 10 mm, the fine component being clay. Improvement in packing can be brought about by keeping the size ratio constant and varying the proportions of the components in order to obtain an optimum mass fraction corresponding to the size ratios. Alternatively, improvement in particle packing can be obtained by varying the size ratio for fixed proportions of coarse and fine components.
The purpose of the present work was to study the influence of mixing/milling on sintering and technological properties of anorthite-based porcelainised stoneware. The influence of mixing/milling on the resultant material has been investigated using sintering study, flexural strength measurement, X-ray diffraction (XRD) and scanning electron microscopy (SEM) techniques.
2. Experimental procedure
The effect of prolonged mixing/milling on sintering and technological properties of anorthite-based porcelainised stoneware material was assessed by simulating, at a laboratory scale, the stoneware making process and by characterizing the finished products. Ball mills, employed for mixing/milling in this study, are frequently used to carry out mixing as well as particle size reduction. The grinding media, consisting of alumina balls, are effective in breaking up agglomerates, which are present in the constituent powders and which form during mixing.
To facilitate the mixing/milling study, five batches having the same composition, details of which is given elsewhere [1], were prepared and each batch was mixed/milled in a ball mill for 3, 12, 24, 48 and 96 h separately. In order to prepare
1 kg of batches, appropriate amounts of starting materials
were weighed and wet mixed/milled for the determined times in a 5 l porcelain pot containing 70 alumina balls of rv15 mm in diameter. After mixing/milling, some slurry was withdrawn for particle size analysis and the remaining slurry was transferred to the plastic container and oven dried at rv110 8C. The particle size distributions of mixed/milled batches were analysed by a Malvern Mastersizer m+Ver.
2.15 model laser diffraction particle size analyser. Dried
powder cakes were broken up to form a powder, which was then granulated by first spraying with a fine mist of water droplets and then by agitating the damp powder. To produce the test samples, moist granules of the starting materials, were uniaxially pressed by means of a hand-operated hydraulic press at 30 MPa being maintained for 60 s. While sintering study was carried out on disc test-pieces the flexural strength study was conducted on rectangular bars. The unfired bars had dimensions of 7 mm x 75 mm x approximately 4 mm. The unfired discs were 50 mm in diameter and approximately 3.5 mm thick. Both discs and rectangular bars were fired at temperatures from 1150 to
1325 8C with soaking time of 3 h, in the Nabertherm chamber kiln, to see how the materials densify. The rate of heating was kept at 3 8C/min.
The densities after firing were determined from the
volume of discs and their masses. The firing shrinkages were determined by measuring the diameter of discs before and after sintering. The water absorption of the sintered discs was measured by a boiling water method. The flexural strength of sintered test bars was measured with an electronic universal tester (Model 5569, Instron ltd.) by a three point bending fixture with a lower span of 50 mm and crosshead speed of 1 mm/min, based on ASTM standard C1161-90. The surface condition of tested specimens was as-sintered. The number of specimens used varied between eight and ten for each sintering temperature.
The crystalline phases present in the sintered samples were identified by XRD technique. For XRD, powdered form of sintered samples were scanned from 2u = 5 to 708, at a scanning speed of 18/min, using a RIGAKU 2200 DMAX
M.U. Taskiran et al. / Ceramics International 32 (2006) 325–330 327
diffractometer (with Cu Ka radiation, l = 0.154 nm) at
40 kV and 40 mA. The diffractometer was calibrated with a silicon standard before use. The JCPDS cards listed on the diffraction patterns were used to identify crystalline phases. For SEM observations, specimens were polished using 6, 3,
1 mm diamond pastes after grinding with silicon carbide powders as abrasive and lubricated with water. A Phillips XL30 SFEG scanning electron microscope (operating at
20 kV) was used for microstructural examination of samples
with secondary electron images (SEI) used predominantly.
3. Results
The effect of mixing/milling time on the particle size distribution is shown in Fig. 1. It can be seen that 3 h mixing/ milling time was not enough to obtain a proper bimodal distribution of the components that is achieved when the mixing/milling time exceeds 12 h. Only for 3 h mixing/ milling there seems to be a third component at around 20 mm. Since this component was rather weak comparing with others, it is considered that the particle size distribution for 3 h mixing/ milling is bimodal. The analyses show bimodal distributions presenting two maximum points, which are centred at around
0.4 and 4 mm. The ratio between coarse and fine particle sizes is around 10. Further increase of mixing/milling time does not significantly alter the ratio between coarse and fine particle sizes but varies the proportions of the coarse and fine components. Keeping the size ratio constant and varying the proportions of the components could be useful for bringing about the improvement in the packing by obtaining an optimum mass fraction corresponding to the size ratios.
Fig. 1. The effect of mixing/milling time on the particle size distribution of mixed batches.
To observe the effect of mixing/milling time on densification behaviour, specimens of anorthite-based bodies mixed/milled for different times were sintered in the temperature range from 1150 to 1325 8C with 25 8C intervals. Densification was monitored by measuring variables such as bulk density, firing shrinkage and water absorption. The effect of mixing/milling time on densifica- tion behaviour of bodies can be clearly seen in Fig. 2a–c. where the sintering results of all the bodies being separately mixed/milled for 3, 12, 24, 48 and 96 h were plotted together. As a general observation upon heating, bulk density and firing shrinkage of all the bodies continued to increase, reached a maximum and then decreased. This relationship is typical behaviour of ordinary porcelains. It
Fig. 2. (a) Densification behaviour, (b) shrinkage behaviour, (c) water absorption behaviour of anorthite-based porcelainised stoneware bodies that were mixed/milled for different durations prior shaping.
328
M.U. Taskiran et al. / Ceramics International 32 (2006) 325–330

Fig. 3. SEM micrographs of sintered samples of anorthite-based porcelainised stoneware bodies that were mixed/milled for different durations and sintered at
1200 8C.
can be seen from Fig. 2a and b that with the increase of mixing/milling time the level of bulk density and firing shrinkage increased significantly and the sintered bodies reached their maximum bulk density and firing shrinkages in lower temperatures and maintained these values for narrower sintering temperature ranges.
Fig. 2c shows the variation in water absorption with sintering temperature for the bodies mixed/milled for different times. It is evident from the results that with progressive increase of mixing/milling time up to 48 h there was a beneficial effect on reducing water absorption of specimens. Water absorption values and the temperature to reach a minimum water absorption level gradually decreased with the increase of mixing/milling time of batches. The ISO
13006 standard prescribes a maximum water absorption value of 0.5% for the porcelainised stoneware materials. As shown in Fig. 2c, already at 1200 8C the water absorption of the sintered samples produced from a batch being mixed/ milled for 48 h becomes almost zero indicating that the fired body achieves the standard’s requirement.
SEM analysis indicated differences between the mor- phology of the porosities in sintered samples of bodies being mixed/milled for different times and sintered at 1200 8C (Fig. 3). The sample of 3 h mixed/milled batch (Fig. 3 a) contains both isolated large pores and generally inter- connected fine pores in large numbers as a result of incomplete densification. It was observed that the finer pores appear to have been removed from the material when the mixing/milling time was increased. All the samples produced from batches that were being mixed/milled for prolonged times contain essentially isolated large pores. The size and number of large pores (Fig. 3b–d) are also reduced with the increase of mixing/milling time up to 48 h. However, in the sample of 96 h mixed/milled batch (Fig. 3e) the number of pores appears to increase again.
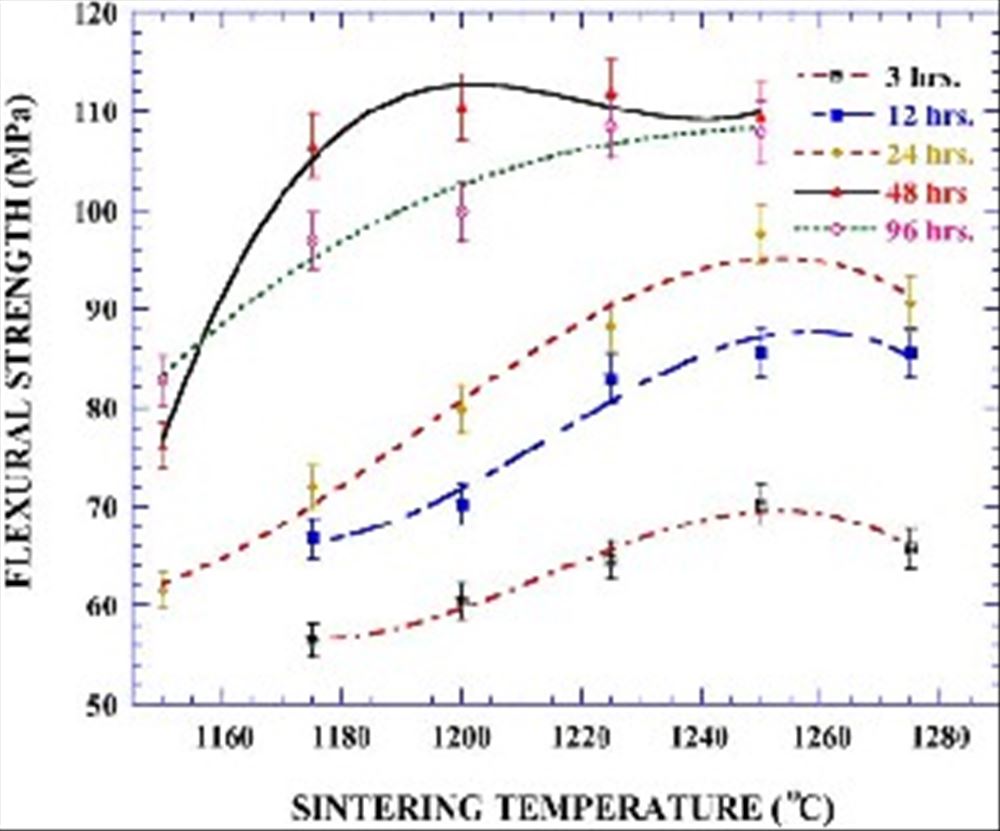
Fig. 4 shows the flexural strength behaviour of bodies that mixed/milled for different times sintered in the temperature range from 1150 to 1275 8C. The flexural strength of the material increased with an increase in sintering temperature. Upon further heating, the flexural strength reached a
M.U. Taskiran et al. / Ceramics International 32 (2006) 325–330 329


Fig. 6. XRD traces of anorthite based porcelainised stoneware bodies with mixing/milling time showing anorthite, corundum and cristobalite forma- tion labelled as A, C and Q, respectively.
Fig. 4. The flexural strength behaviour of anorthite-based porcelainised stoneware bodies that were mixed/milled for different durations as a function of sintering temperature.
maximum and then decreased with a corresponding decrease in density. The mixing/milling time has a greater effect on flexural strength up to a certain threshold level. The flexural strength increased with milling time up to 48 h. While the sintered samples of 3 h mixed/milled batch exhibit about
70 MPa maximum strength value at 1250 8C, sintered samples of 48 h mixed/milled batch have about 110 MPa maximum strength value at 1200 8C. Further mixing/milling did not have the same beneficial effect on the flexural strength: it was rather the reverse. In this study the flexural strength of the sintered material was found to be proportional to its bulk density (Fig. 5).
Fig. 6 shows the X-ray diffraction traces of bodies sintered at 1250 8C as a function of mixing/milling time. The crystalline phases identified in all the specimens are

Fig. 5. The relationship between flexural strength and bulk density in anorthite-based porcelainised stoneware bodies mixed/milled for different durations.
anorthite, cristobalite and corundum, the former being the major phase. Some glassy phases are also present. The peak intensities of crystalline phases did not significantly change with the increase of mixing/milling time indicating that the increase of mixing/milling time did not significantly change the degree of crystallinity at 1250 8C. However, specimens sintered at this temperature showed marked increase in their flexural strength with the increase of mixing/milling time. These results clearly indicate that reduction in porosity plays major role for the increase in flexural strength.
4. Discussion
The mixing/milling of anorthite-based porcelainised stoneware bodies for different times prior shaping changes the particle size distribution. Relatively short time, e.g. 3 h of mixing/milling the batch was not enough to obtain a bimodal distribution of the components, however when mixing/milling time reaches about 12 h or more it was then possible to obtain a proper bimodal distribution and the ratio between coarse and fine particle sizes was a little above 10. This large size ratio would allow the larger voids between the coarser particles to be filled with smaller particles as was suggested by the Furnas model. The mixing/milling time that exceeds 12 h did not significantly change the size ratio between coarse and fine particles but changed the proportions of the coarse and fine particles in the favour of fines. However, keeping the size ratio constant and varying the proportions of the components up to a certain level was useful for the improvement in the densification and strength behaviour of the anorthite-based porcelainised stoneware. Microstructural studies indicated that the increase in mixing/milling time up to 48 h improved the densification resulting in higher bulk densities by making the pores smaller and homogeneously distributed in the microstructure. Further, mixing/milling up to 96 h was not beneficial for the improvement in densification even though this longer mixing/milling treatment did not change the size ratio however changed the proportions further between the coarse and fine particles in the favour of the latter.
330
M.U. Taskiran et al. / Ceramics International 32 (2006) 325–330
The increase in densification up to 48 h is due to the improvement in the particle packing by obtaining an optimum mass fraction corresponding to the size ratios. The smaller size particles among larger ones can increase the powder reactivity and can produce the liquid phase. In viscous sintering, when a liquid phase forms it endeavours to minimise its interfacial energy. It does this by pulling the body together reducing the pore space between the particles. The use of smaller sized particles mean that the pore radii are small and so there is a high driving pressure for pore closure.
The flexural strength was found to be proportional to relative density and inversely proportional to number and size of closed porosity. Generally, pores must be eliminated because the bending strength of ceramics decreases exponentially with the increase in porosity.
In the present investigation, maximum flexural strength as well as maximum bulk density was obtained at a water absorption value of almost zero and both values decreased with further increase in closed porosity values. The decrease in density after reaching maximum is attributed to
‘‘bloating’’ (i.e. pore volume expansion), which arises from higher pressures (at high temperatures) of gases entrapped within closed pores. Gases like nitrogen, carbon monoxide and carbon dioxide are not soluble in the viscous liquid and they would stop pores shrinking when the gas pressure rises. The formation of viscous liquid is the onset of vitrification and consequent densification. The vitrification temperature is defined as the temperature at which the apparent porosity becomes almost zero. If, on reaching the point where the open pore system is closed, gases, which evolve from the breakdown of impurities, can no longer escape, bloating can be severe and the bulk density can rapidly decrease.
5. Conclusion
In conventional ceramic processing of this kind, it is almost impossible to have a structure without closed porosity; however, as it is done in this study, if mixing/ milling provides both a proper bimodal particle size distribution, near to that predicted by the Furnas model, and an optimum mass fraction corresponding the size ratios as well as reduces the particle size it would eventually be possible to end up with minimum amount of closed porosity in the microstructure.
In laboratory processing condition 48 h of mixing/milling impart the best sintering and technological properties to newly developed anorthite-based porcelainised stoneware and any further mixing/milling does not improve the sintering and technological properties any more. The powder density of fired stoneware was measured as
2.80 g/cm3 and the measured maximum bulk density after mixing/milling 48 h and firing at 1200 8C is about 2.40 g/ cm3. Among all, this mixture also showed better strength characteristics, attributed to its relatively lower volume percent of small size closed porosity. The relationship between bulk density and flexural strength is found to be linear. The maximum flexural strength obtained with 48 h mixed/milled body of anorthite based porcelainised stone- ware is about 110 MPa which has a potential to be used in heavy-traffic areas subjected to mechanical stresses.
References
[1] M.U. Taskiran, N. Demirkol, A. Capoglu, A new porcelainised stone- ware material based on anorthite, J. Eur. Ceram. Soc. 25 (2005) 293–
300.
[2] A. Capoglu, M.U. Taskiran, Processing of ultra white porcelainized stoneware, Key Eng. Mater. 264-268 (2004) 1495–1498.
[3] G. Tari, J.M.F. Ferreira, A.T. Fonseca, O. Lyckfeldt, Influence of particle size distribution on colloidal processing of alumina, J. Eur. Ceram. Soc. 18 (1998) 249–253.
[4] J.S. Reed, Batching and mixing, in: Introduction to the Principles of
Ceramic Processing, John Wiley & Sons, 1988 (Chapter 17).
[5] P.F. Messer, Uniformity in processing, Brit. Ceram. Soc. Trans. J. 82 (1983) 156–162.
[6] Y. Lin, R.L. Bowland, P.F. Messer, Assessment of the uniformity of composition, in: J.S. Moya, S. deAza (Eds.), Processing of the Advanced Ceramics, Soc. Esp. Ceram. Vidr., Madrid, 1986.
[7] Y. Lin, P.F. Messer, Mixing prior to calcinations, Brit. Ceram. Soc.
Trans. J. 86 (1987) 85–90.
[8] P.F. Messer, Batching and mixing, Ceramics and Glasses, Engineered
Materials Handbook, vol. 4, ASM International, 1991, pp. 95–99. [9] W.E. Lee, Y. Iqbal, Influence of mixing on mullite formation in
porcelain, J. Eur. Ceram. Soc. 21 (2001) 2583–2586.
[10] H. Ries, The importance of mixing technology for the preparation of ceramic bodies, Interceram 3 (1971) 224–228.
[11] P.M.C. Lacey, Developments in the theory of particle mixing, J. Appl.
Chem. 4 (1954) 257–268.
[12] J.C. Williams, Mixing of particulate solids, Chapter 16, in: V.W. Uhl, J.B. Gray (Eds.), Mixing: Theory and Practice, vol. 3, Academic Press, 1986.
[13] C.W. Clump, Mixing of solids, in: V.W. Uhl, J.B. Gray (Eds.), Mixing: Theory and Practice, vol. 2, Academic Press, 1967 (Chapter
10).
[14] G.L. Messing, G.Y. Onada, Inhomogeneity packing density relations in binary powders, J. Am. Ceram. Soc. 61 (1978) 1–5.
[15] R.K. McGeary, Mechanical packing of spherical particles, J. Am.
Ceram. Soc. 44 (1961) 513–522.
[16] A.B. Yu, N. Standish, Porosity calculation of multicomponent mix- tures of spherical particles, Powder Tech. 52 (1987) 233–241.
[17] J.M.F. Ferreira, H.M.M. Diz, Effect of the amount of deflocculant and
powder size distribution on the green properties of silicon carbide bodies obtained by slip casting, J. Hard Mater. 3 (1992) 17–27.
[18] G. Tari, J.M.F. Ferreira, O. Lyckfeldt, Influence of magnesia on colloidal processing of alumina, J. Eur. Ceram. Soc. 17 (1997)
1341–1350.